- A+
含硅化合物的合成因其在有机合成、药物化学和材料科学中的广泛应用而受到广泛关注。烯烃的广泛易得使得从烯烃合成有机硅成为该领域中最重要的课题之一, 而烯烃的碳硅化反应则可一步快速组装更复杂的有机硅化合物。尽管烯烃的碳硅烷化反应研究已经取得了巨大的成就, 但是迄今为止, 这些研究仅得到1,2-碳硅化产物。此外, 通过硅-Heck启动的烯烃碳硅化的反应仍未有报道。武汉大学高等研究院阴国印教授课题组和湖北工程学院生物医学材料工业技术研究所朱磊教授合作报道了镍/铜双金属协同催化的非活化末端烯烃的1,n-芳硅化反应(n=2-5), 以题为“Synergistic Ni/Cu catalyzed migratoryarylsilylation of terminal olefins”发表于Science Bulletin。该反应用Suginome试剂(PhMe2SiBpin)作为硅源, 以硅-Heck反应启动, 镍金属物种在烷基链上迁移至特定位置后与和芳基溴化物发生作用, 从而得到烯烃1,n-芳硅化产物。该反应条件温和、底物范围广泛、且具有极好的区域选择性。实验证明铜金属物种对于Suginome试剂的转金属过程具有至关重要的作用。本文分析了烯烃与芳基之间碳链的长度对反应的影响, 对反应机理进行了阐释。同时新合成了一系列吡啶环上具有不同电性的吡啶噁唑啉配体, 并对配体的空间位阻和电性对反应的影响分别做了分析与讨论。
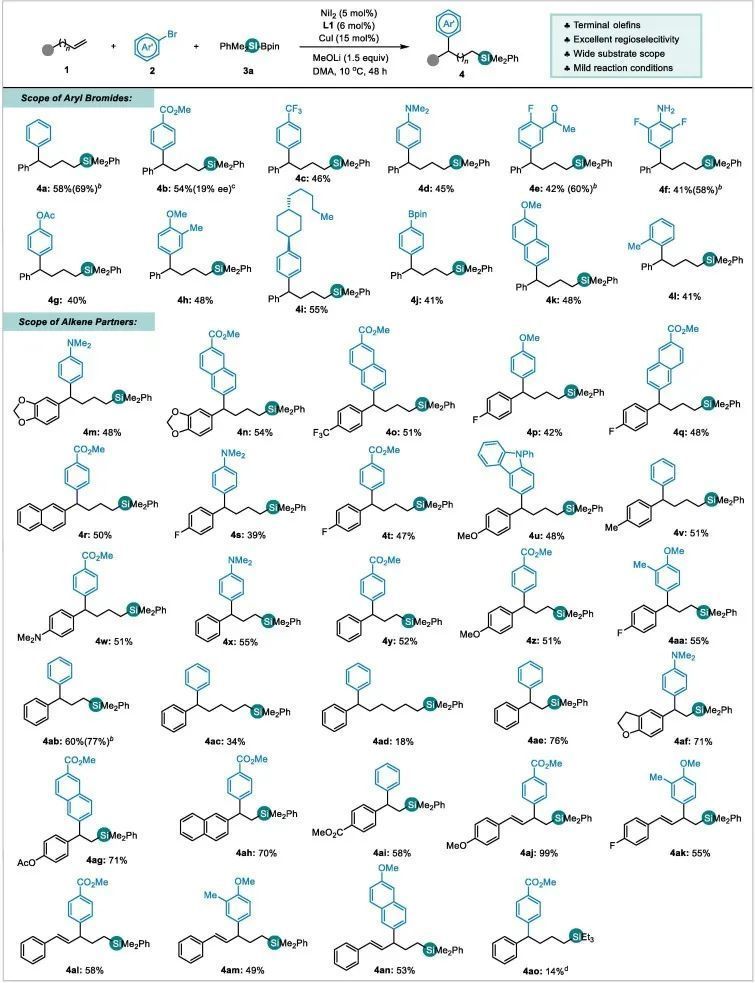
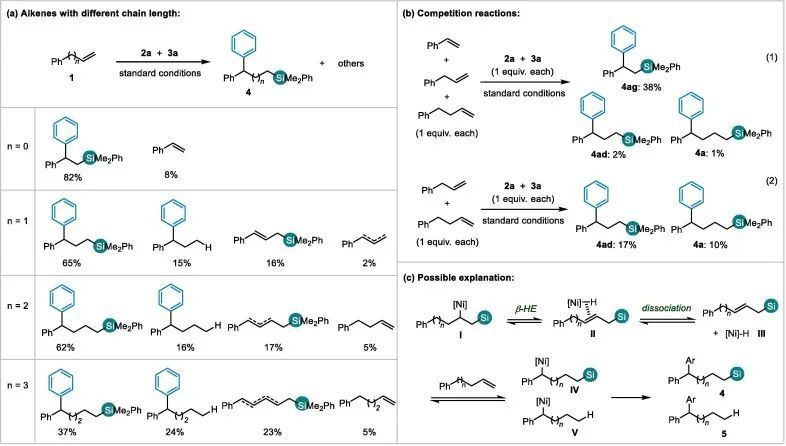
图文速览 Fig. 1. Strategies on alkenes to organosilanes. Table 1. Reaction optimizationa) Entry Derivation from standard conditions Yield of 4a (%) b) 1 No change 62(58) c) 2 NiBr2 instead of NiI2 36 3 NiCl2 instead of NiI2 33 4 Ni(acac)2 instead of NiI2 0 5 CuCl2 instead of CuI 18 6 CuBr instead of CuI 59 7 CuCN instead of CuI 0 8 MeONa instead of MeOLi Trace 9 MeOK instead of MeOLi Trace 10 tBuOLi instead of MeOLi 0 11 LiOH instead of MeOLi 39 12 1,4-dioxane instead of DMA 0 13 THF instead of DMA 0 14 DMF instead of DMA 26 15 Room temperature instead of 10 oC 42 Entry Ligand Yield of 4a (%) Yield of 5a (%) Yield of 6a (%) Yield of 7a (%) Yield of 8a (%) Yield of 9a (%) Recovery of 1a (%) 1 No 8 12 13 25 17 9 0 2 L1 62 16 17 0 0 0 5 3 L2 5 28 26 1 0 24 9 4 L3 48 19 16 7 0 0 9 5 L4 15 28 37 2 0 0 1 6 L5 34 32 20 3 0 0 3 7 L6 34 24 18 5 0 0 11 8 L7 3 6 13 15 10 5 15 9 L8 0 0 0 0 0 0 only 10 L9 28 18 19 16 0 0 10 11 L10 58 18 17 1 0 0 4 12 L11 0 0 0 0 0 0 only Fig. 2. Study toward the role of copper salt (a-c) and proposed mechanism (d). Fig. 3. Scope of the arylsilylation reaction. Reaction conditions: NiI2 (5mol%), L1 (6 mol%), 1 (0.4 mmol), 2 (0.7 mmol), 3a (0.6mmol) and LiOMe (0.6 mmol) in DMA (3 mL), 10 °C, 48 h. Isolated yields. (a) Theyield of the reaction on a 0.2 mmol scale, and with additional Ph3SiH(0.2 mmol), 2 (0.2 mmol) and LiOMe(0.2 mmol), rt, 24 h. (b) ee of the reaction with (S)-L1 instead ofL1. (c) 3a replaced by Et3Si-Bpin (3b), 30 °C, 48 h. Fig. 4. Study toward the effect of chain length.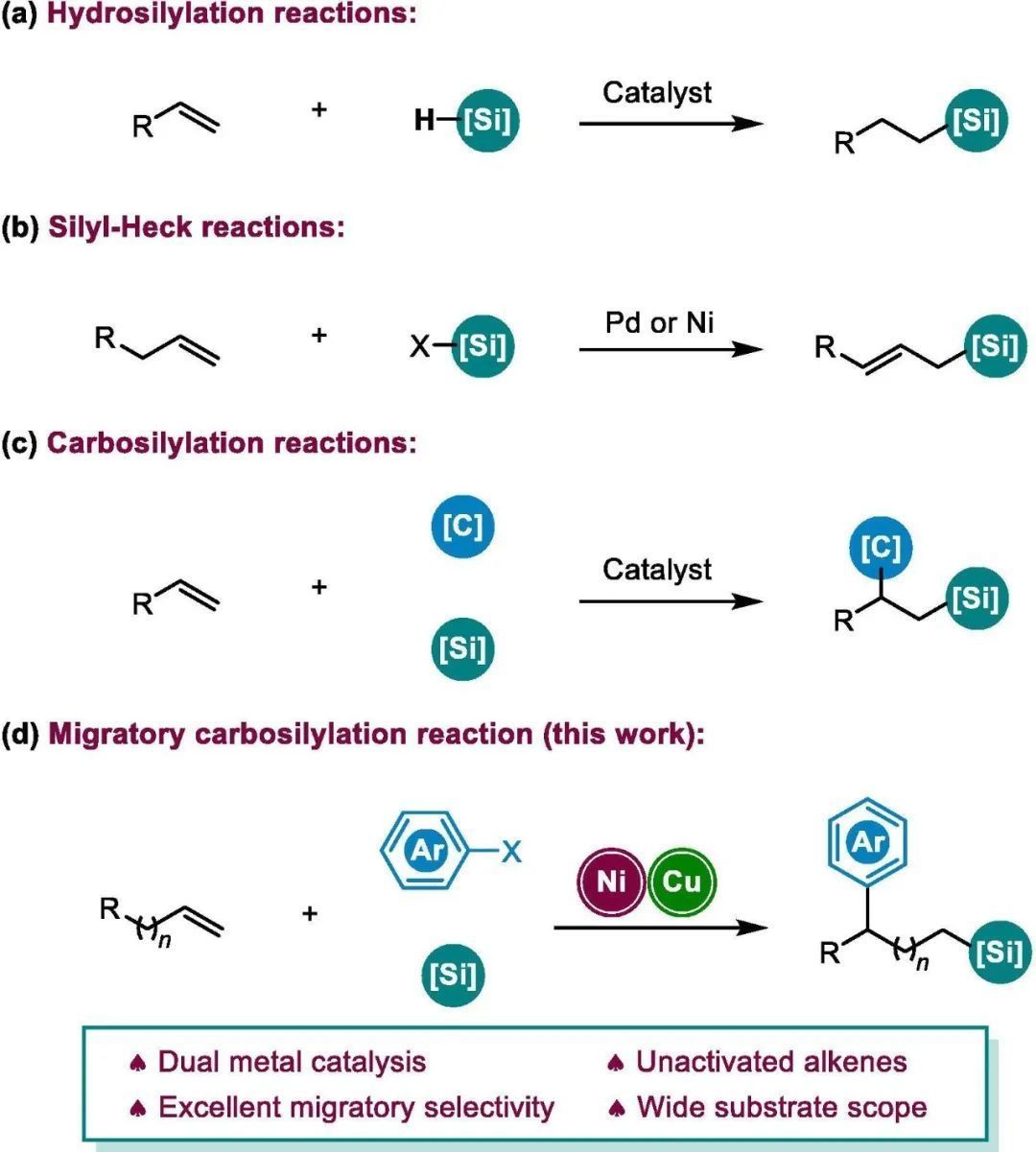

(a) Reactionconditions: NiI2(5 mol%), L1 (6 mol%), 1a (0.2mmol), 2a (0.35 mmol), PhMe2SiBpin(3a, 0.3 mmol) and LiOMe (0.3 mmol)in DMA (1.5 mL), 10 °C, 48 h. (b)GC yield withnaphthalene as internal standard. (c) The number in parenthesis is the isolatedyield on a 0.4 mmol scale.

(a) Reaction conditions: NiI2 (5 mol%), L1 (6 mol%), 1a (0.2 mmol), 2a (0.35 mmol), PhMe2Si-Bpin (3a, 0.3 mmol) and LiOMe (0.3 mmol) in DMA (1.5 mL), 10 °C,48 h. GC yield withnaphthalene as the internal standard.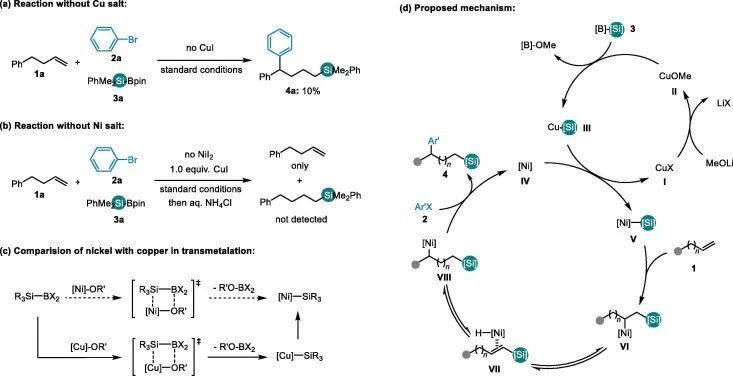


目前评论: